8. EudraCT Import/Export
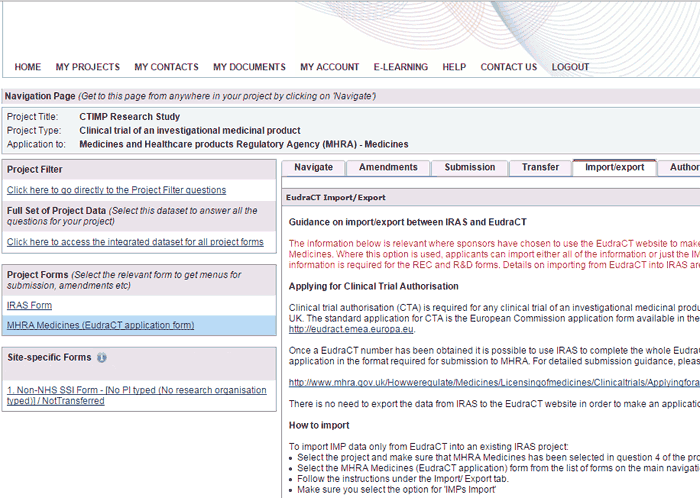
The Import / Export tab is only available on the MHRA Medicines (EudraCT application) form which you will need if you are applying for a Clinical Trial Authorisation (CTA) for a clinical trial of an investigational medicinal product (CTIMP).
For CTIMPs with sites in both the UK and EU, the Import / Export tab allows you to import either all of the information or just the IMP information from the European Clinical Trials Database (EudraCT) into IRAS.
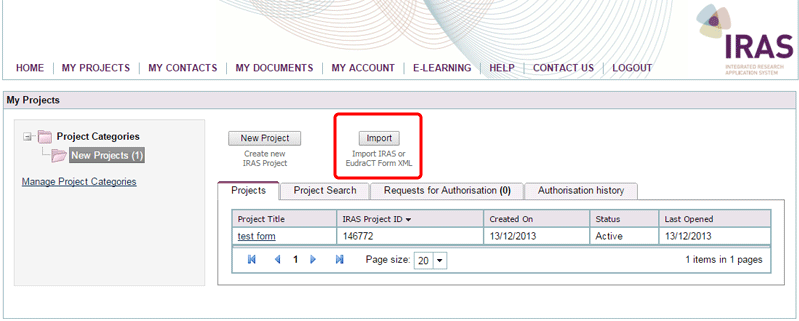
You can access the same functionality from the Import button on the ‘My Projects’ page.
Using IRAS in combination with EudraCT, for CTIMPs with sites in both the UK and EU
- Applicants can complete a EudraCT form through the EudraCT website first and then import the information into IRAS.
- To import information from EudraCT you can either:
- To export data from IRAS to EudraCT:
- You can complete the information in IRAS and export all the information prepared in IRAS to EudraCT if you wish. Please note that this is not necessary in order to make an application to MHRA as you can create the appropriate XML file and printout from the Submission tab. This option may be useful to transfer data to colleagues preparing applications to other Competent Authorities.
- To export information from IRAS, select the MHRA Medicines (EudraCT application) form from the list of forms and follow the instructions under the Import/ Export tab.
- Further guidance can be found on the IRAS Help pages available from the main menu.
Points to remember
- For CTIMPs with sites in both the UK and EU, once a EudraCT number has been obtained it is possible to use IRAS to complete the whole EudraCT dataset and to save the application in the format required for submission to the MHRA.
- Submission guidance is available from the Submission section of this guide and from the MHRA.
- There is no need to export the data from IRAS to the EudraCT website in order to make an application to the MHRA.