Amendments
Each section below provides more information on how to make an amendment in the system:
You can create an amendment for any project with an 'Authorised' status.
You should start by downloading the Amendment Tool from standard IRAS and completing it. The functionality within the tool will show the amendment type and category and also which bodies will need to review it.
To create the amendment in new IRAS, go to 'My Projects'. Select the IRAS ID of the project and from the project overview page, select 'New amendment' in the top right-hand corner of the screen.
Enter the sponsor amendment reference number (this is the number assigned to the amendment by the sponsor), sponsor amendment date, and select whether this is a substantial or non-substantial amendment. Take care to enter this information accurately as it will populate directly into letters issued by the regulators.
It is important that all information you enter about your amendment matches the supporting documentation, in particular the Amendment Tool. Any inconsistencies may result in your amendment being rejected and you'll need to create a new one.
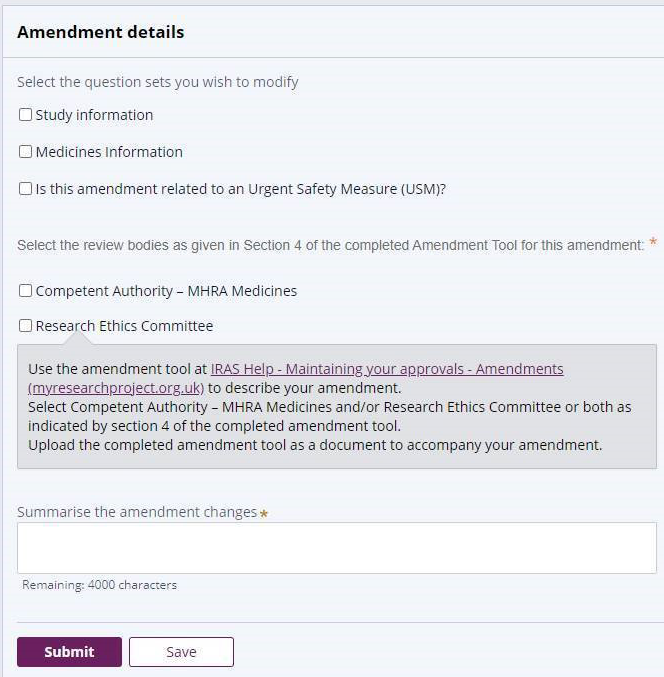
You'll need to select the amendment type by choosing one of the following options:
For a substantial amendment, select one of the following options :
- Chief Investigator (CI) (for amendments to change the CI)
- Sponsor Group (for amendments to either change the sponsor, the sponsor's legal representative or the organisation conducting the work on behalf of the sponsor in IRAS)
- Administrative (if you are only changing the contact details for the CI, sponsor, sponsor contact or sponsor legal representative)
- Project information (for any other type of substantial amendment not covered by the above. This option also allows you to indicate whether the amendment relates to an Urgent Safety Measure)
For a non-substantial amendment, select one of the following options:
- Non-substantial
- Non-substantial no study wide review required
- Extend study end date (if your amendment will extend the study end date you should select this type of amendment).
Important: You will need to submit certain amendments separately. The following amendment types cannot be combined with any other changes: 'Chief Investigator', 'Sponsor Group', 'Administrative' and 'Extend Study End Date'.
For example, to update a CI's contact details, you should submit an 'Administrative' amendment. Do not include any other changes with the submission. Do not submit the change as part of a different amendment type.
When you're ready, select 'Create amendment'. This will take you to the amendment dashboard. This will look different depending on the type of amendment you're making. You'll only be presented with questions that are relevant to the amendment type you've chosen.
The process for completing this section is very similar to making the initial submission. Some of the information may be populated from the original application depending on how the application was submitted. Work through each question ensuring that you complete all fields. Also make sure that any pre-populated information remains correct. Any information you enter here should also match the Amendment Tool. For example, if an amendment summary is required, you can use the text you have supplied on the amendment tool.
If you're submitting an amendment to extend the end date of your study, you should follow the instructions above in the 'Creating an amendment' section of this guidance. However, you'll not need to submit any documents (including the amendment tool) with your amendment. Instead in new IRAS you'll be asked to enter the new end date, after which you can submit your amendment.
Once you submit the amendment you'll receive a confirmation email that gives instructions on how you should proceed including how you should share the amendment, and the completed amendment tool, with participating NHS/HSC organisations.
For substantial amendments to combined review studies that were previously given an unfavourable opinion by a REC, the Sponsor or Chief Investigator can submit a modified amendment that takes account of the concerns raised by the REC. Before you submit a modified amendment you should complete a new amendment tool, ensuring you identify it as a modified amendment within the tool, and prepare any supporting documentation (for example a cover letter detailing how the concerns raised by the REC have been addressed).
Once these are ready you can submit your amendment in new IRAS by following the same process you used for submitting your original amendment. When you are preparing to submit your modified amendment you should ensure the category of amendment you select in new IRAS matches what the amendment tool categorises it as.
We would also recommend you consider entering text in the 'Sponsor Amendment Reference number' field that identifies it as a modified amendment (for example 'Modified Amendment 01'). This will help to alert the REC that it needs to be reviewed within 14 days.
Use this type of amendment to submit a change of sponsor or to change organisational access in the system.
If you're submitting an amendment to change the sponsor, but you're not changing which organisation will be managing the work on behalf of the sponsor (the Sponsor Delegate Organisation), then you will not need to update the sponsor group for the project in new IRAS as part of this amendment.
If you're preparing an amendment to change the organisation managing the work on behalf of the sponsor in the system (the Sponsor Delegate Organisation) you should update the sponsor group in new IRAS when you're preparing this amendment. You can do so by selecting the 'Amend Sponsor Group' button and then selecting the pencil icon.
We recommend that if you're preparing a sponsor group amendment you contact the HRA Service Desk (service.desk@hra.nhs.uk) for support in preparing and completing your submission.
'Chief Investigator', 'Sponsor Group' and 'Administrative' amendments can only be made one at a time. You'll have to complete and receive approvals for any previous amendments before you can start a new 'Chief Investigator', 'Sponsor Group' or 'Administrative' amendment.
Unlike the amendment types listed above, it is possible to have more than one 'Project Information' amendment in progress at a time.
To do this, any previous amendments you've created need to be received and validated with the status of 'Pending regulator decision', before further amendments can be made.
Multiple 'Project Information' amendments cannot be made at the same time if:
- more than one amendment contains changes to the same document (this does not apply to uploading a new amendment tool)
- more than one amendment involves changes to the same question set in IRAS
In the amendment dashboard there will be a section titled 'Project documents'. Within this section you'll be able to upload supporting documentation relevant to your amendment. The process for uploading documents here is the same process you would follow when submitting documents as part of your initial application. For a description of the process visit Adding Information: Uploading supporting documentation for a description of this process.
When you have added all the supporting documents, and completed the other relevant sections of the amendment dashboard, if you select 'Next' at the bottom of the page this will take you to the page where you can verify and submit your amendment.
On the following page, select 'Verify answers'. If any items are outstanding these will be flagged in a pink box at the top of the screen. If there are outstanding items then you'll need to open the section it refers to and look through it in order to identify the outstanding item and address it before continuing.
When flagged items have been resolved you can continue to select 'Request review'. This will route the amendment to the Sponsor or Sponsor Delegate's account for them to check and submit for review. This will appear for them under 'My Tasks' - 'My Organisational Tasks', with an outstanding task to confirm submission.
If you need to delete the amendment you can do this by selecting the 'Actions' button in the top right of the screen and selecting 'Delete amendment'. Deleting an amendment cannot be reversed, and you'll need to supply a reason for deleting. Amendments that have already been submitted cannot be deleted.
An amendment workaround is currently in place for combined review projects prepared before 31 December 2020. For these projects the Study information and Medicines Information sections will not be complete. This is because this version of IRAS was not in use at the time the application was prepared.
When making an amendment to these studies you will need to avoid activating the system verification. Triggering this verification would mean you would need to complete the entire dataset.
For the workaround start by creating the amendment. From the amendment details page de-select all question sets. You should deselect them regardless of whether they are applicable to your amendment. You can then supply details of the amendment by attaching a completed amendment tool and relevant supporting documentation in the documents section. Uploading documentation will not activate the validation.